Pharma Consulting: Digital Transformation with Impact
Navigating Complexity. Driving Innovation. Ensuring Compliance.
Pharmaceutical companies today operate in a highly regulated and fast-evolving environment. At Orise, we support process manufacturers in managing complexity by aligning digital transformation with operational excellence and compliance requirements. Our pharma consulting services combine deep domain knowledge, robust methodology, and cutting-edge technologies to future-proof manufacturing, laboratory, and quality processes.
Whether you’re launching a new facility, scaling production, optimizing validation, or adapting to new regulations—our experts help you turn strategy into execution with measurable impact. We deliver tailored pharmaceutical consulting services designed to address your most pressing challenges in manufacturing, laboratory digitalization, and pharma compliance.
What Challenges Do We Help Solve?
Pharma companies face growing pressure to scale efficiently, remain compliant, and innovate faster than ever. Orise helps clients respond to these demands through an integrated approach. We support the seamless setup of new production sites, fast-track speed to market, and build digital manufacturing systems that are scalable and secure. As a leader in digital transformation in pharma, we ensure readiness for evolving regulatory frameworks, reduce risk of license loss, and digitize process documentation.
Our approach enables smart, flexible, and compliant operations through technology innovation—driving lean validation processes, automation, and data-driven decision-making. From early design to execution, Orise is a trusted partner for pharmaceutical manufacturing solutions that help you stay competitive and resilient.

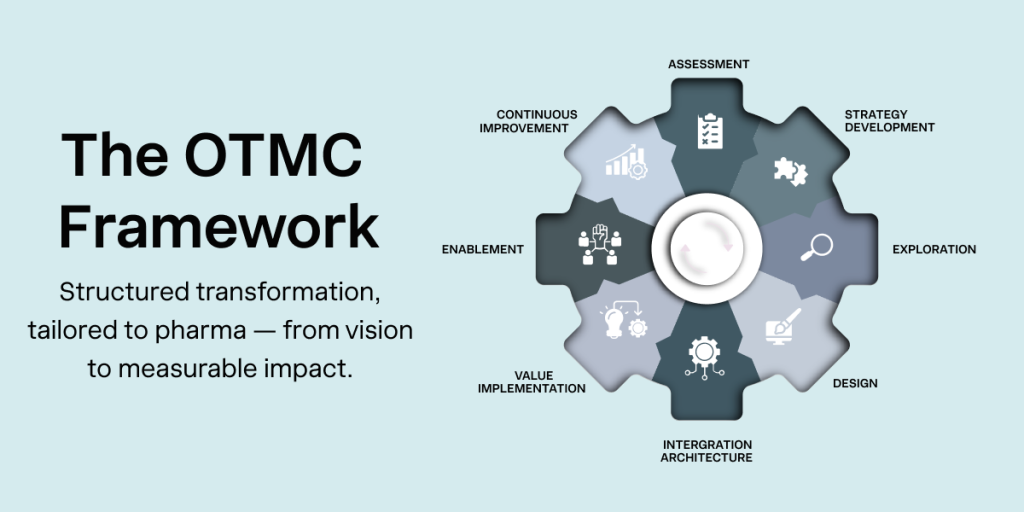
The Orise Consulting Framework: From Vision to Value
Our consulting approach is built on the OTMC Framework (Orise Transformation Methodology & Consulting)—a structured, industry-specific model that guides clients through every phase of their digital and compliance journey.
We work across three strategic dimensions:
- Quality & Compliance: GXP/CSV assessments, validation strategy, and GMP-aligned concept creation
- Digital Transformation: Integration assessments, solution design, digital manufacturing strategy for pharma, MBR excellence, and project execution
- Technology Innovation: Smart manufacturing roadmaps for pharma, technology process integration, and AI validation services
This framework supports six core transformation stages:
- Assessment
- Strategy Development
- Exploration & Design
- Integration Architecture
- Value Implementation
- Enablement & Continuous Improvement
Why Choose Orise?
Deep Industry Expertise
We bring decades of experience across pharmaceutical manufacturing, laboratory operations, and quality assurance. Our consultants understand the intricacies of regulated environments and deliver pharma quality control solutions that are both innovative and compliant with GxP, GMP, and CSV/CSA standards.
End-to-End Transformation Service
From initial assessments to execution and post-implementation enablement, we cover the full transformation lifecycle. Our OTMC methodology ensures a structured, transparent, and value-driven approach across all project phases — supporting everything from regulatory compliance to pharmaceutical validation and compliance solutions.
Compliance & Quality Focus
In a sector where compliance is non-negotiable, Orise ensures you stay inspection-ready. We help clients reduce the risk of non-conformities, license loss, and regulatory penalties through robust validation strategies and precise documentation, partnering with you on every aspect of pharma compliance consulting and GMP consulting services.
Integrated Tech & Process Consulting
We don’t just implement tools—we integrate them into your workflows. Our consultants work across system layers (MES, LIMS, DCS, Data Historian) to ensure seamless data flow, smart process control, and scalable digital architectures. Whether you need pharma IT and automation consulting or advanced laboratory automation in the pharmaceutical industry, we deliver solutions that fit your environment.
Innovation-Driven Solutions
We bring the future of pharma to your doorstep: AI-powered review systems, digital batch records, intelligent validation engines, and scalable modular systems. Tools like Audit Trail Review, eStreams, and GxP Report Generator help clients accelerate digitalization with confidence, supporting LEAN manufacturing consulting for pharma and promoting sustainable improvement.
Independent, Client-Centric Approach
Orise is vendor-neutral. Our only focus is what works best for your business. We guide you in selecting, evaluating, and implementing the right solutions—without bias or hidden agendas.
Proven Track Record
We’ve supported global pharma clients in launching new facilities, redesigning validation strategies, integrating MES & LIMS systems, and future-proofing operations. Our success stories reflect our commitment to pharmaceutical industry consulting and to delivering measurable results on complex, high-impact projects.


