eStreams
Connect your fragmented automation into one unified DataOps layer for faster and fully compliant operations.
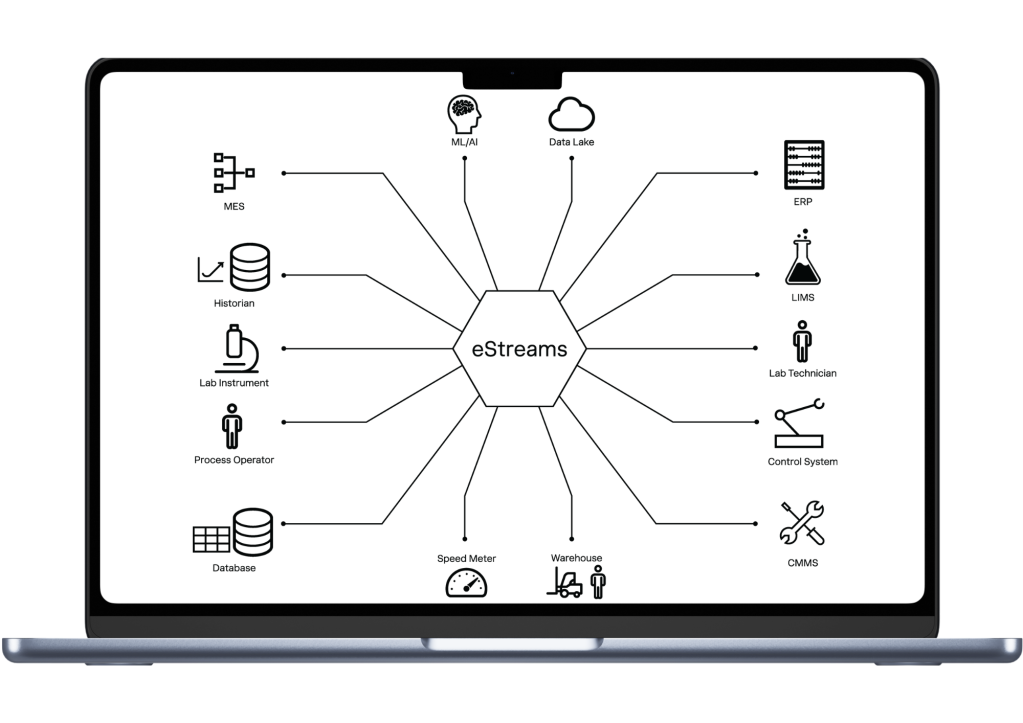
In regulated manufacturing, integration between systems like MES, LIMS, ERP, and process control is often manual, code-heavy, and hard to validate or scale.
Orise eStreams turns these fragmented connections into a workflow-driven DataOps platform designed for Life Science operations.
Orise eStreams orchestrates data exchange, validation, and approvals across your automation and enterprise layers, ensuring data integrity, traceability, and audit readiness — all within a single, qualified environment.
With visual configuration, version control, and GxP compliance built in, Orise eStreams enables faster digital projects and sustainable Pharma 4.0 readiness.
From Custom Code to Configurable, Validated Workflows
Orise eStreams replaces one-off, code-heavy integrations with a structured, auditable workflow framework that simplifies how data moves between your systems — from connection to execution.
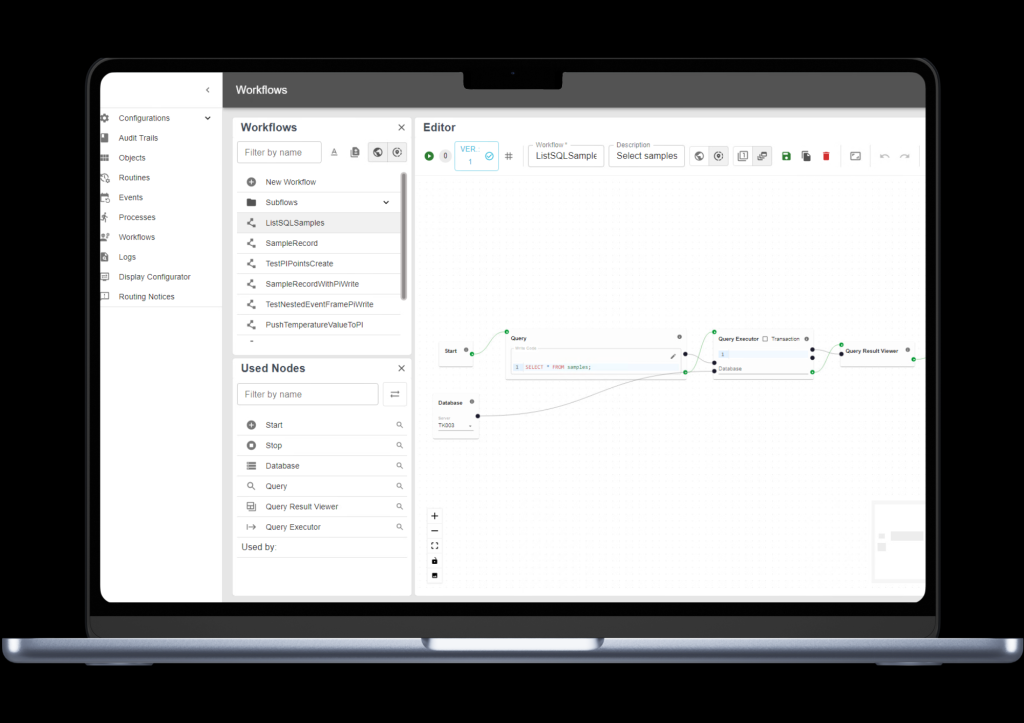
Instead of developing and validating separate scripts for each interface, eStreams from Orise lets you design, approve, and execute integration logic through visual workflows that are fully version-controlled and traceable.
Each step, from connecting to data sources to executing workflows in production, follows a clear, compliant process that ensures data integrity, audit readiness, and repeatability across your operations.
Enhanced Functionalities for Optimal Performance
Orise eStreams bridges business goals and technical execution — giving leadership real-time visibility, centralized control, and scalability, while empowering engineers with precision, configurability, and compliance by design.
- Workflow Engine: Build, approve, and execute integration logic through a no-code/low-code, version-controlled environment with full audit trails.
- System Connectivity: Connect PLCs, SCADA, MES, LIMS, ERP, and Cloud systems via native OPC UA, REST, MQTT, and SQL — ensuring secure, real-time data flow.
- Validation-Ready Deployment: Validate once and the re-using many times accelerating CSV processes and minimizing qualification overhead.
- Edge & VLAN Deployment : Deploy close to production assets or in isolated, validated networks without compromising data integrity.
- Reusable Workflow Libraries: Standardize logic across equipment and sites, reducing project time and ensuring consistent execution.
- Human Interaction Nodes: Combine operator input with automated workflows for flexible, compliant operations.
- Custom Node and AI Service Integration: Integrate AI models or microservices within validated flows to drive smarter decisions and predictive performance.
- ISA-95 Data Modeling: Standardize structure across workflows using models aligned with ISA-95 levels (site, area, line, equipment…).
Whether you’re leading digital transformation or engineering compliant data flows, Orise eStreams gives you one platform to connect, validate, and scale — securely and efficiently.
Why Choose Orise eStreams?
Configurable over coded
Design and modify integration logic visually, without custom code development or hidden scripts.
Validate once, reuse often
Create versioned, approved workflows that can be redeployed across lines, equipment, and sites for faster scale-up.
Audit-ready by design
Every change, approval, and execution is automatically logged, ensuring full traceability and inspection of readiness.
Connects everything
From PLCs and SCADA to MES, LIMS, historians, and cloud APIs, eStreams provides end-to-end data orchestration.
Human + system orchestration
Combine operator input with automated logic for flexible, compliant process control.
Edge-ready deployment
Run close to the process, even in VLAN-isolated or validated on-prem environments, for secure operations.
Accelerates digital projects
Standardize integration through reusable workflow templates, reducing engineering and validation time.
Supports AI : Integrate model inference and retraining as part of validated data flows to enhance process optimization and quality analytics.


